| Home | Authors | Publications | Lectures | Links |
V.V. Serdobintseva, A.F. Danilyuk, D.V. Kalinin
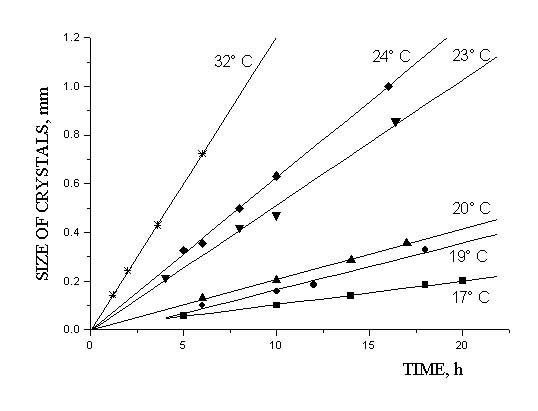
Kinetics of the supramolecular crystallization of monodisperse spherical silica particles (MSSP) suspended in diethyl ether with a concentration of structural units close to the phase transition point has been studied for the first time. It was found that the linear crystal growth rate depends on temperature and the process activation energy is 25 kcal/mol. The linear law of crystal growth is associated with diffusion of the structural units on the interface.
Linear growth of the supramolecular crystals at 17–32°C
(MSSP are suspended in a mixture of diethyl ether and 23% ethyl alcohol;